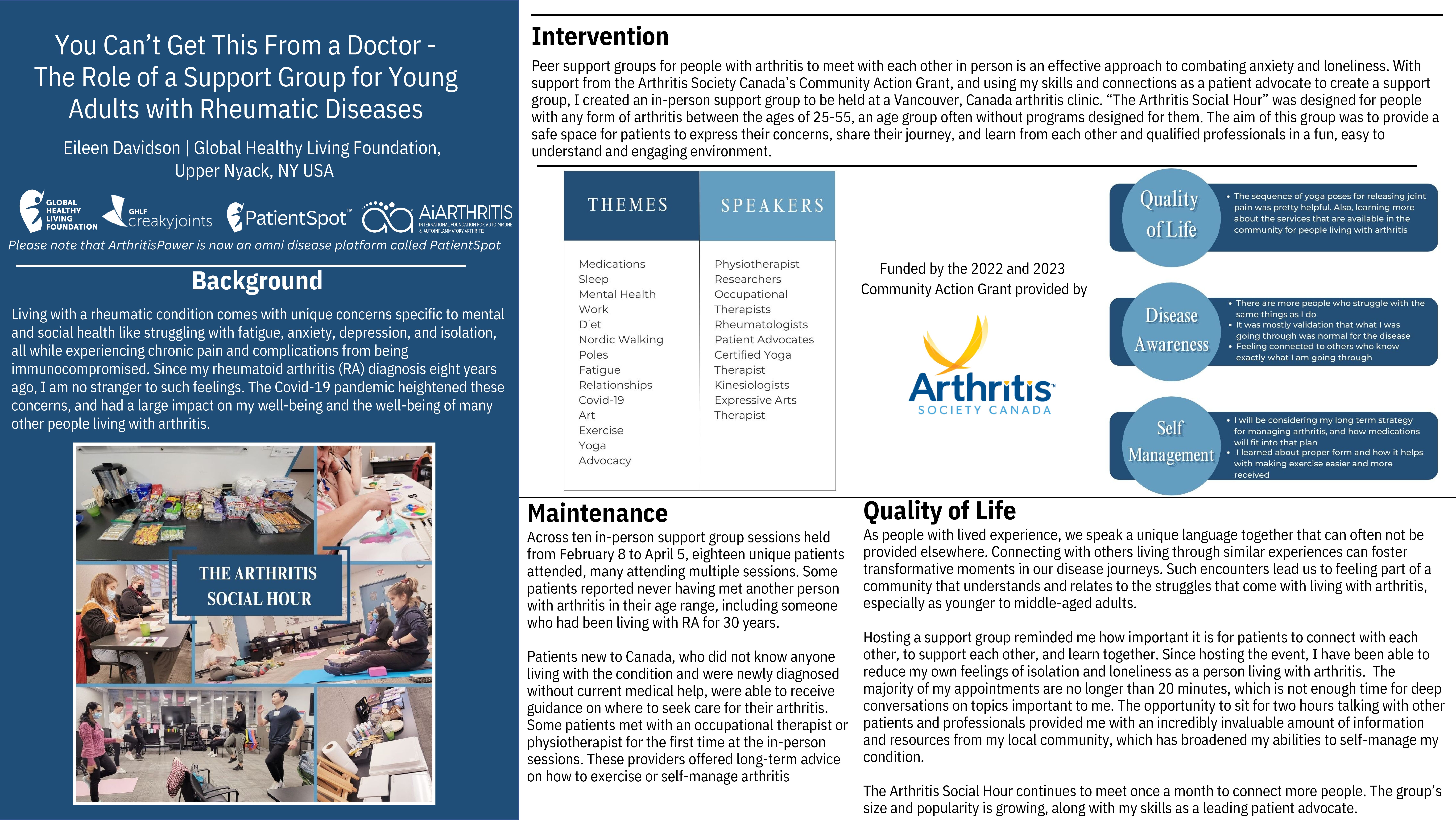
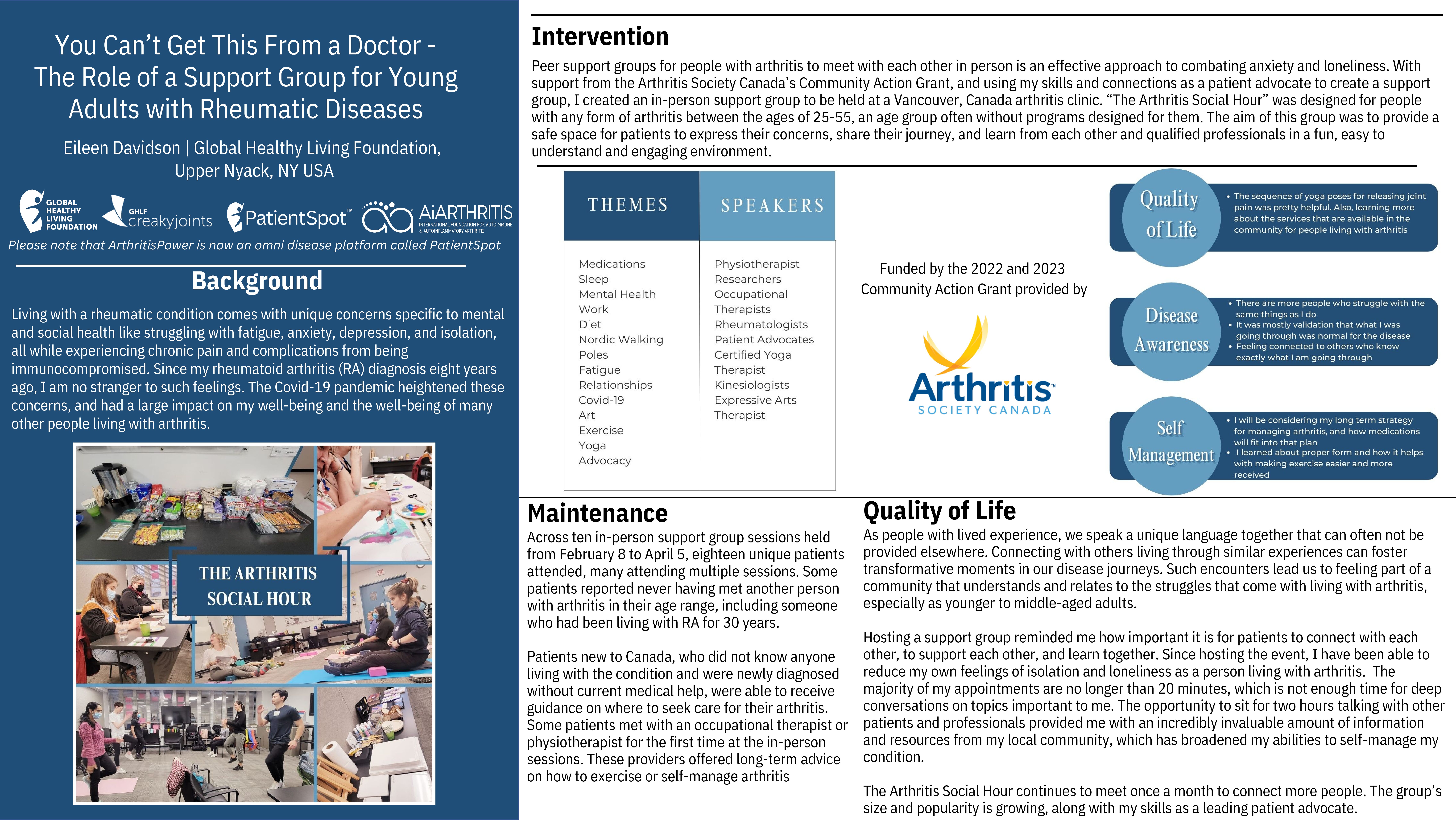
Learn more about our FREE COVID-19 Patient Support Program for chronic illness patients and their loved ones.
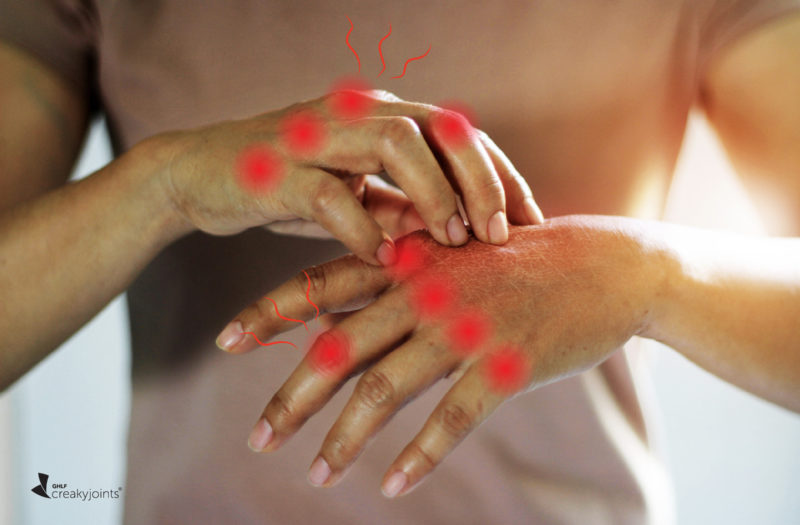
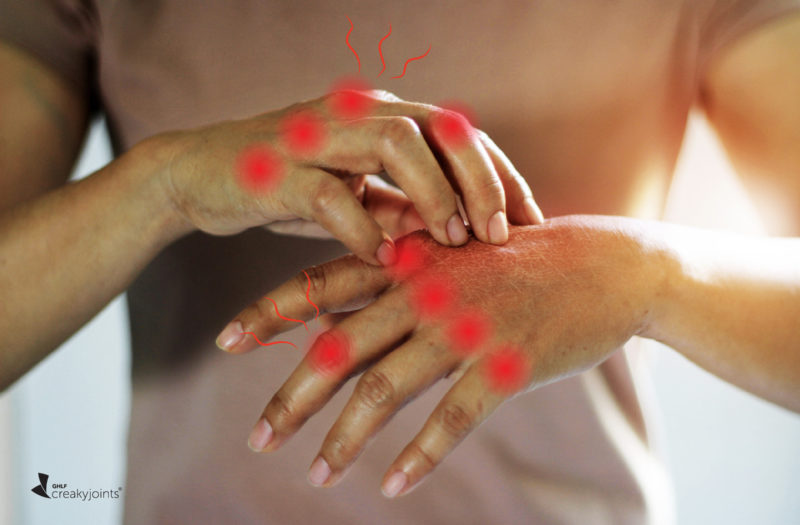
When the COVID-19 vaccine was first rolled out in December 2020, many people with rheumatic diseases worried about the potential side effects. Specifically, many were concerned about whether the vaccine would lead to a disease flare. This was, and still is, an understandable concern, given that rheumatic disease patients were not included in the initial COVID-19 vaccine trials.
Since then, some studies have been done to assess the risk of flares in rheumatic diseases patients. One study, which was presented at the European Alliance of Associations for Rheumatology (EULAR) 2021 Virtual Congress in June, found that adverse events like flares are rare and mild in rheumatic disease patients who receive a COVID-19 vaccine.
And a recent study from the Johns Hopkins University School of Medicine further supports these findings.
For the study, which was published in the journal Arthritis & Rheumatology, researchers recruited people with rheumatic disease who were on immunomodulatory therapy and had received both doses of either the Pfizer or Moderna vaccine between December 16, 2020 and April 15, 2021.
Participants had a variety of rheumatic diseases, including inflammatory arthritis, systemic lupus erythematosus (SLE), and overlap connective tissue disease. The majority were being treated with combination therapy (which means taking multiple medications), while others were being treated with either disease-modifying antirheumatic drugs (DMARDs) or biologic therapy.
Patients were asked to complete a questionnaire detailing local and systemic reactions they experienced within a week of each vaccine dose, and one month after the second dose. Local reactions included pain, redness, and swelling. Systematic reactions included fever, fatigue, headaches, chills, vomiting, diarrhea, and myalgia (muscle aches). Patients were asked to rank the reactions based on the impact they had on daily activities. The scale included:
- No interference with daily activity
- Some interference with daily activity
- Prevention of daily activity
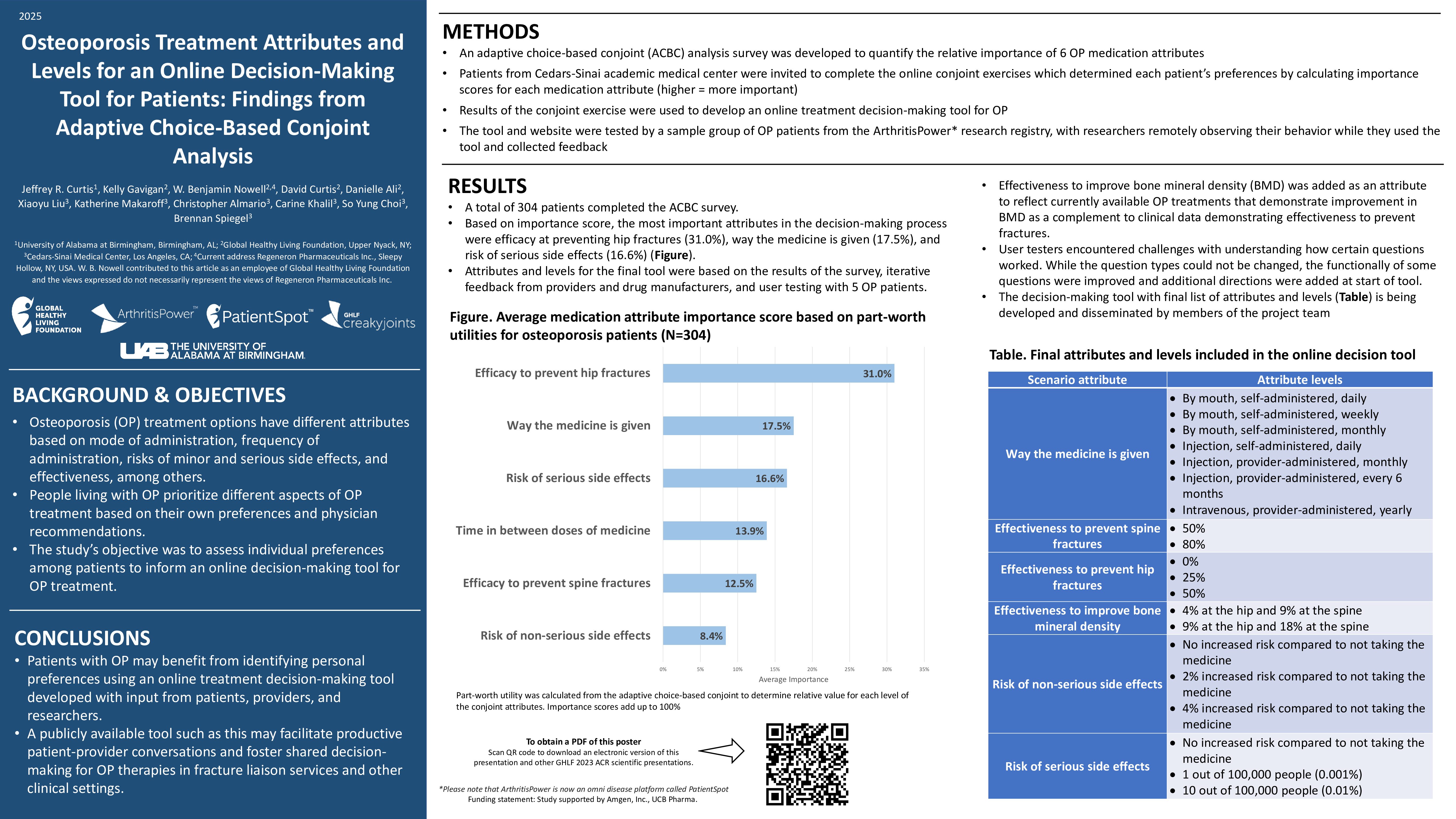
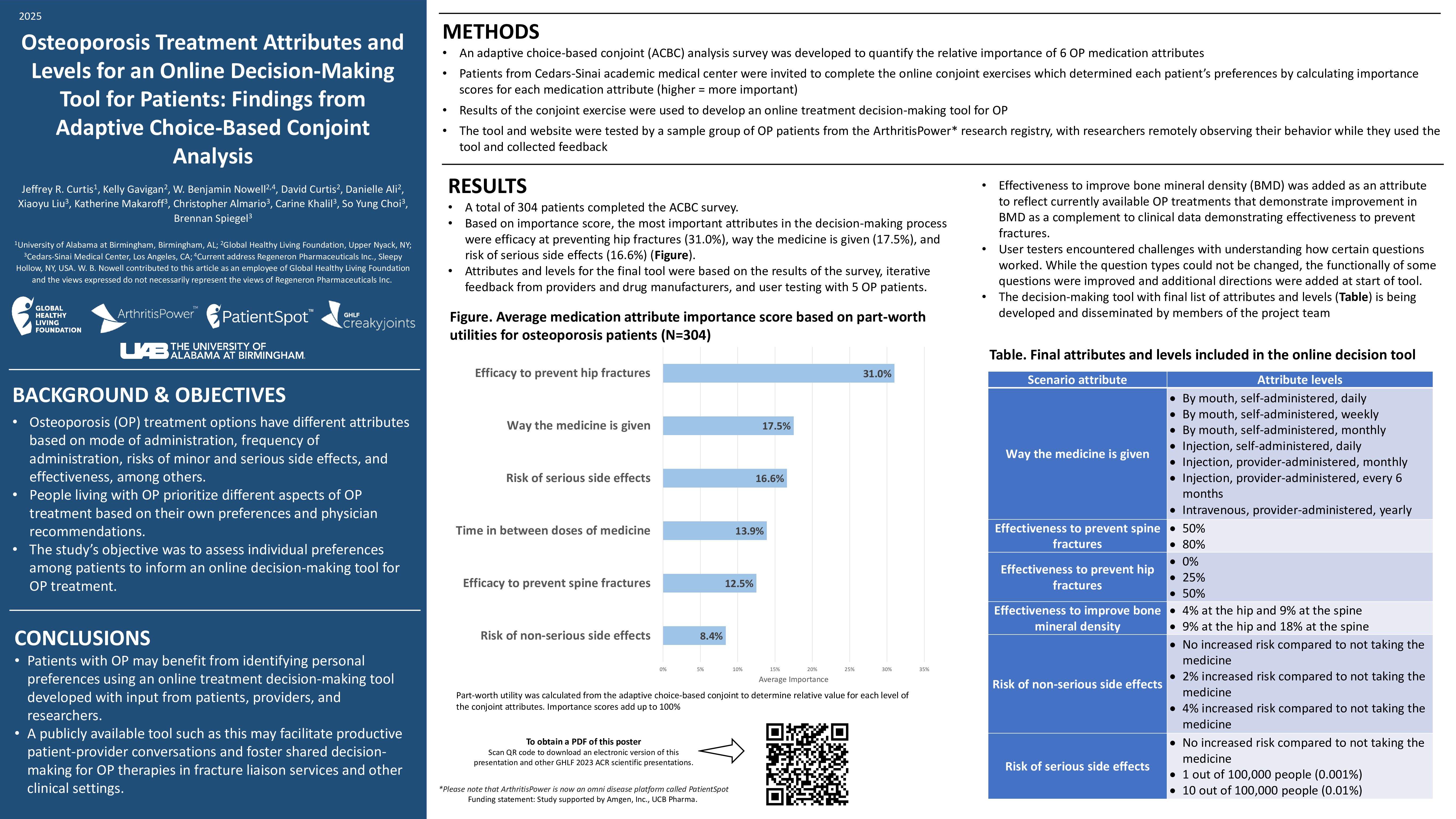
Of the 1,377 patients who participated in the survey, only 151 (11 percent) said they experienced flares that required treatment following vaccination. Of those, 60 percent said the flares occurred after the second vaccine dose. No one reported severe flares.
Although local and systemic reactions were common, very few patients reported symptoms that prevented daily activities. The most common reported adverse reactions were:
- Pain at the injection site
- Fatigue
- Headache
- Muscle aches and pains
- Chills
Researchers found factors associated with a post-vaccine flare included being on a combination therapy and having a flare within the six months prior to being vaccinated. There was also a positive association between previous COVID-19 infection and post-vaccination flares.
“These early, reassuring results may ameliorate concern among patients and inform critical discussions regarding vaccine hesitancy or refusal,” researchers wrote.
Health care providers encourage autoimmune and immunocompromised patients to get a COVID-19 vaccine. In its COVID-19 vaccine guidance, the American College of Rheumatology (ACR) states that rheumatic disease patients, including autoimmune inflammatory rheumatic disease patients, “should receive a COVID-19 vaccine.”
This is particularly important as the Delta variant spreads and the risk of breakthrough infections increases.
Now, of course, rheumatic disease patients on immunosuppressant medication are concerned about getting the third dose of the COVID-19 vaccine, which just became available in the United States. We will be monitoring information about potential side effects and flares, but doctors do not anticipate any issues.
In a press release about the third dose, ACR President David Karp, MD, said “luckily, we have not seen any safety signals in patients with autoimmune and rheumatic diseases from the COVID-19 vaccines, so there should be no concern for the third dose.”
If you have concerns about the COVID-19 vaccine, talk to your rheumatologist.
Get Free Coronavirus Support for Chronic Illness Patients
Join the Global Healthy Living Foundation’s free COVID-19 Support Program for chronic illness patients and their families. We will be providing updated information, community support, and other resources tailored specifically to your health and safety. Join now.
Connolly CM, et al. Disease Flare and Reactogenicity in Patients with Rheumatic and Musculoskeletal Diseases Following Two‐Dose SARS‐CoV‐2 Messenger RNA Vaccination. Arthritis & Rheumatology. August 4, 2021. doi: https://doi.org/10.1002/art.41924.
COVID-19 Vaccine Clinical Guidance Summary for Patients with Rheumatic and Musculoskeletal Diseases. American College of Rheumatology. August 4, 2021. https://www.rheumatology.org/Portals/0/Files/COVID-19-Vaccine-Clinical-Guidance-Rheumatic-Diseases-Summary.pdf
Rheumatology Patients on Immunosuppressive Medications Qualify for Third COVID-19 Vaccine Dose. American College of Rheumatology. August 13, 2021. https://www.rheumatology.org/About-Us/Newsroom/Press-Releases/ID/1159.