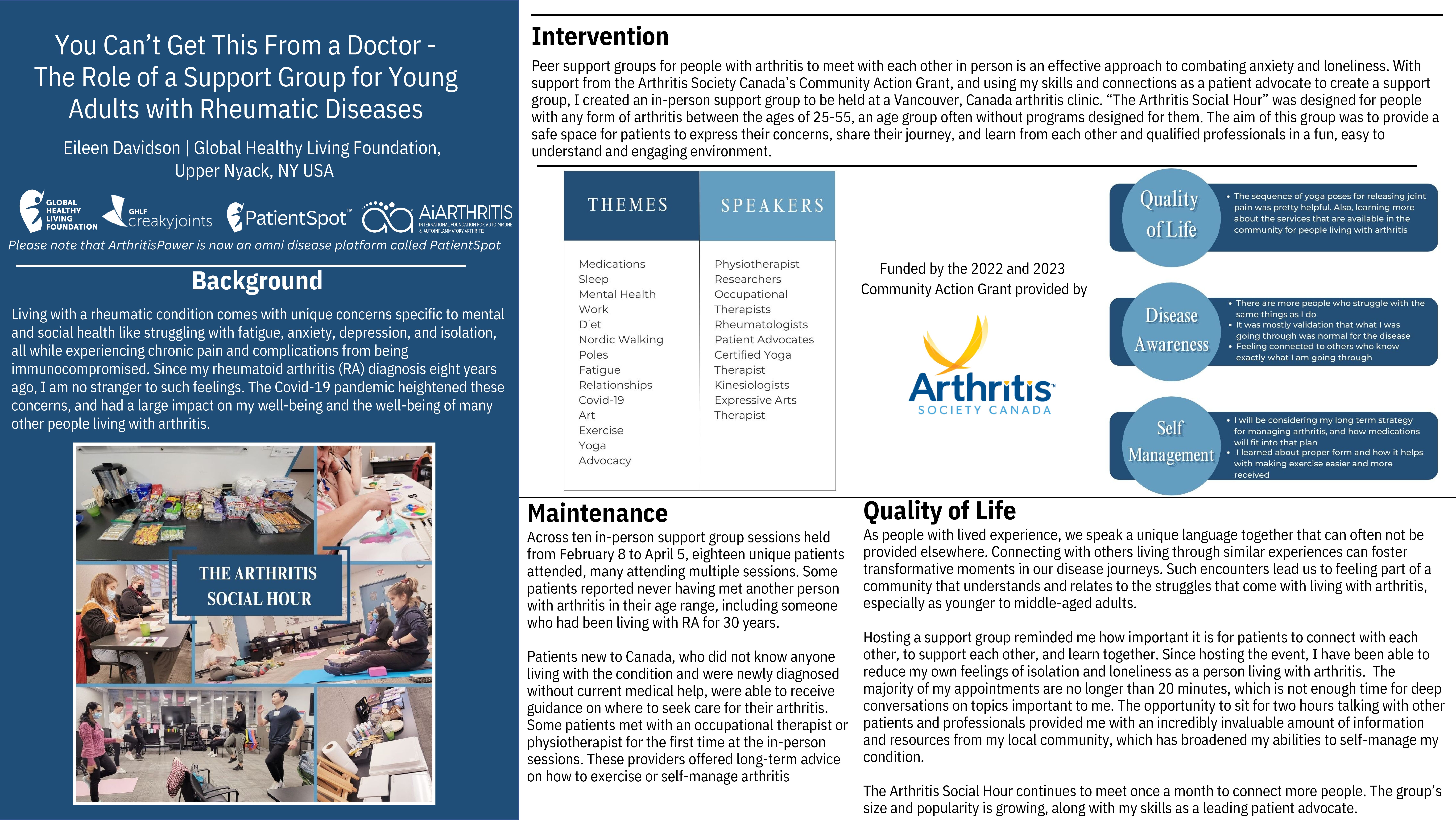
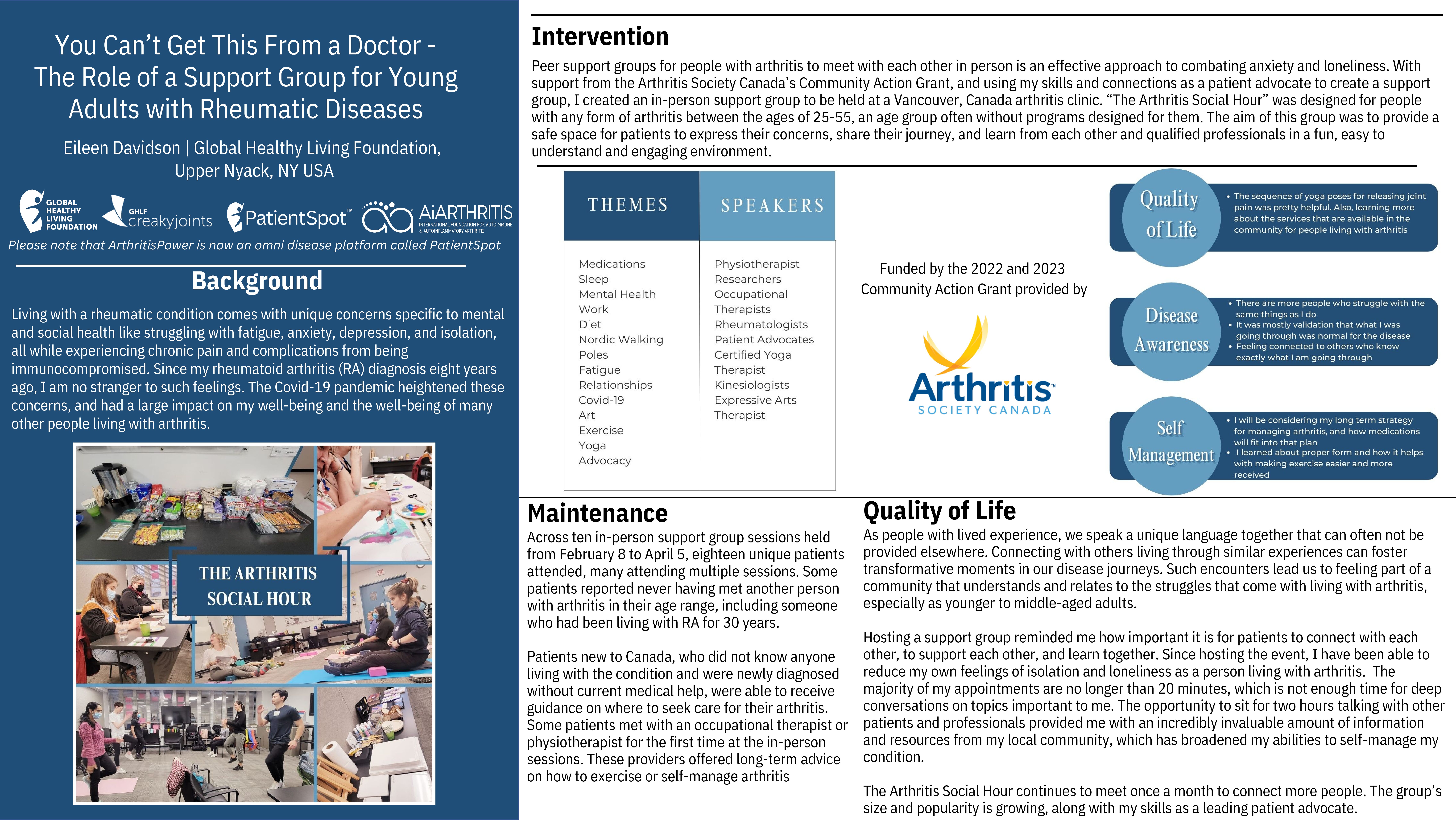
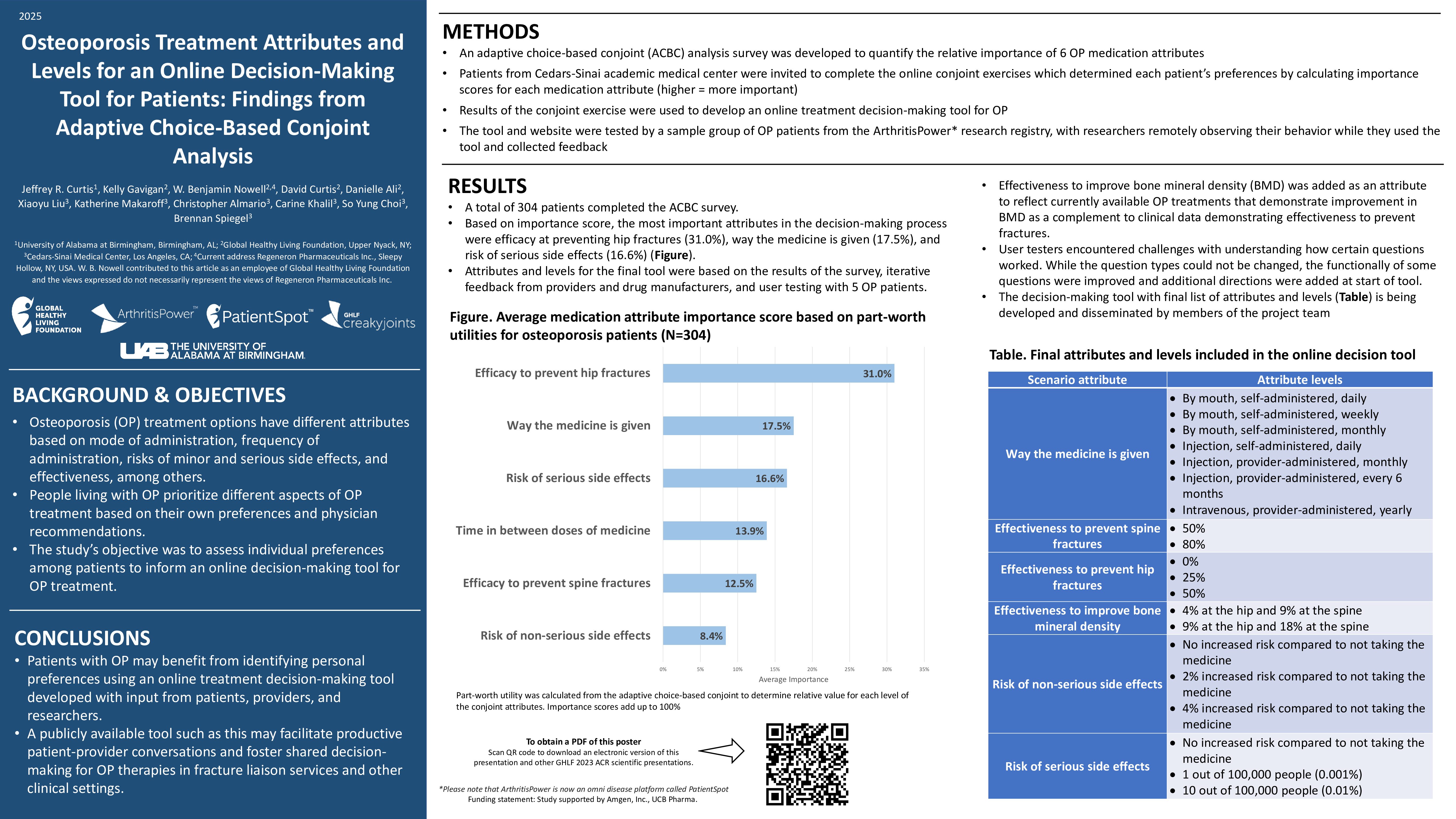
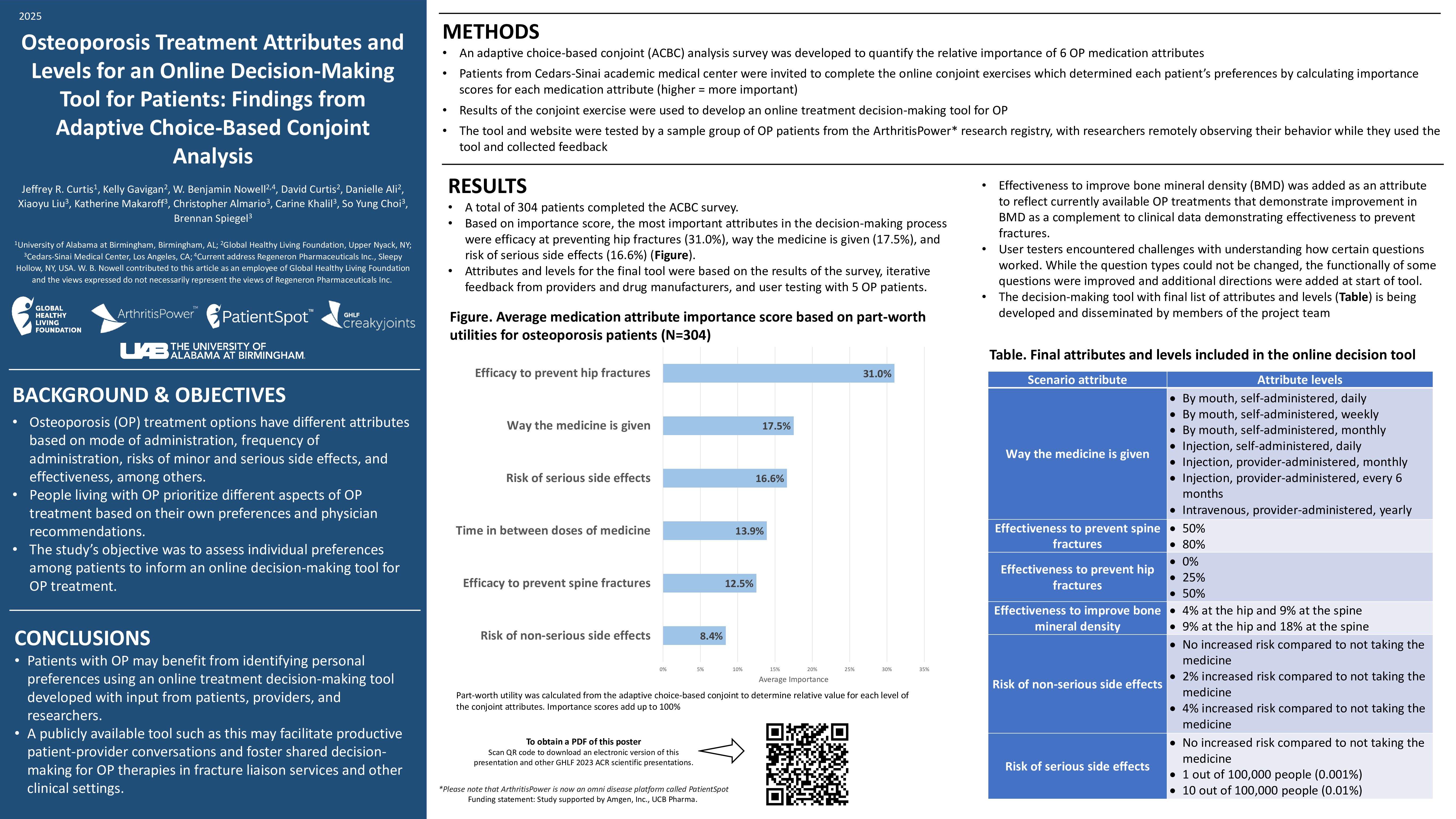
Learn more about our FREE COVID-19 Patient Support Program for chronic illness patients and their loved ones.
On April 13, 2021, the U.S. Food and Drug Administration (FDA) and U.S. Centers for Disease Control and Prevention (CDC) asked states to temporarily halt using the Johnson & Johnson’s COVID-19 vaccine “out of an abundance of caution” after receiving six reports of a severe type of blood clot forming in individuals — all women between the ages of 18 and 48 — after receiving the vaccine (one of which was fatal).
These six cases occurred among nearly 7 million doses of the J&J vaccine administered so far across the U.S.
The blood clots, known as cerebral venous sinus thrombosis (CVST), are considered to be similar to a very rare form of stroke. Cerebral venous sinus thrombosis typically affects about five in 1 million people every year, according to Johns Hopkins Medicine. The blood clots are similar to those reported in several European countries among people who received the AstraZeneca COVID-19 vaccine. They may also be related to some blood clot events that were reported during the Johnson & Johnson vaccine clinical trials, though at the time of the vaccine’s authorization, the clots were not determined to be directly related to the vaccine.
This is a developing story and more information will emerge after the CDC meets this week to review the data more closely.
Here are a few very important facts to know, to start:
1.The blood clots are considered very rare, though they are (and should be) taken seriously.
2. The FDA and CDC are acting out of caution to investigate that this “is a true safety signal” and not just a statistical anomaly, according to epidemiologist Katelyn Jetelina in her Your Local Epidemiologist email.
3. If you already received the Johnson & Johnson vaccine, you should know that the risk of having a blood clot is very low if it’s been more than a month since the vaccine, Anne Schuchat, MD, Principal Deputy Director of the CDC, said in a press conference. For those who got the vaccine in the last few weeks, contact your health care provider if you experience any of the following symptoms of a blood clot:
- Severe headache
- Abdominal pain
- Leg pain
- Shortness of breath
4. If you have a vaccine appointment scheduled for the Pfizer or Moderna COVID-19 vaccine, you should keep it, as officials haven’t detected this blood clot problem with either of these vaccines. For those who have Johnson & Johnson vaccine appointments in the days ahead, officials will be working to reschedule those appointments or administer different vaccines.
5. Until more research is reviewed and analyzed, it’s important to not make assumptions about the safety of the Johnson & Johnson COVID-19 vaccine or assume you won’t be able to or should not get it in the future. Many experts think that further scientific review will demonstrate that the benefits of the Johnson & Johnson vaccine (and, for that matter, of the AstraZeneca vaccine) will continue to outweigh the risks for most people.
In the meantime, it’s important to stay calm, read trusted sources for news and interpretation, and speak to your own health care provider if you have questions about your specific medical situation. We know that people with chronic illnesses often have unique questions and concerns about side effects and we are committed to following this news closely for the Global Healthy Living Foundation community.
To start, we’re sharing some of the best resources and perspectives we’ve seen today that put this issue in context and help alleviate some anxiety.
Physician and researcher Ashish K. Jha, MD, shares why the Johnson & Johnson COVID-19 vaccine pause is important for building vaccine confidence
Calling six events out of nearly 7 million vaccinations “VERY rare,” Ashish K. Jha, MD, Dean of the Brown University School of Public Health, wrote on Twitter that, “confidence is built on having a system that takes adverse events seriously, investigates them, makes data-driven decisions. . . That[‘]s what FDA/CDC doing.” He also added that these sorts of actions should build confidence, rather than hesitancy toward vaccines, as “confidence comes from people believing that we have a vigorous system that takes adverse events seriously.”
Read Dr. Jha’s full Twitter thread here.
Katelyn Jetelina, PhD, of Your Local Epidemiologist answers common questions about the Johnson & Johnson vaccine pause
Dr. Jetelina, who has a PhD in epidemiology and biostatistics, addresses some of the more common concerns about the Johnson & Johnson vaccine and adds important background about what’s also been happening with the AstraZeneca vaccine in Europe, which uses the same technology as the J&J vaccine and may provide some insights as to what will ultimately happen in the U.S.
“After an intense investigation, the European Medicines Agency review board concluded that the benefits of the AZ vaccine far outweighs the risks,” Jetelina wrote. “So, each country who rolled out AZ is doing something different now regarding distribution.” Some are not allowing the AstraZeneca vaccine in younger people (say, under age 55), because the blood clots have only occurred in younger adults. Some aren’t allowing the AZ vaccine at all and some are allowing it for everyone.
Dr. Jetelina notes that “scientists will jointly examine possible links between the vaccine and blood clotting events and determine whether the FDA should continue to authorize use of the vaccine for all adults or limit the authorization.”
Read the full article here and her ongoing coverage here.
Megan Ranney, MD, compares the risk of blood clots with COVID-19 infection and with the Johnson & Johnson vaccine
Using data to support her arguments, Dr. Ranney, an emergency physician at Brown Emergency Medicine, explained on Twitter that in general “the risk of catching [COVID-19] and getting a bad complication is much higher than this very rare adverse event.” She acknowledged that this assumption may not be true for some groups of people but that “science and medicine, like life, is full of weighing risks vs. benefits.”
Dr. Ranney shared that the risk of getting a blood clot with COVID-19 is approximately 20 percent, or 20 in 100. That’s a lot greater than what’s known so far about the numbers associated with the J&J vaccine blood clot risk.
“It is difficult for most of us to accurately judge risk of omission (risk of avoiding vaccines) versus risk of commission (risk of getting vaccines),” she wrote. “We overestimate the latter, to our own — and our community’s — peril.”
Read Dr. Ranney’s full Twitter thread here.
Matthew Herper of STAT News dives into the ‘why’ behind how vaccines could cause blood clots
What could be driving, biologically, the link between the Johnson & Johnson vaccine or the AstraZeneca vaccine and these rare blood clots?
Herper’s article discusses recent studies published in the New England Journal of Medicine that found in some European patients who received the AstraZeneca vaccine, the blood clots were seen in people who had high levels of antibodies to something called platelet factor 4. “That doesn’t explain why a vaccine is causing the immune system to produce those antibodies, or whether other vaccines might do the same,” Herper wrote. “But it provides a first step toward explaining the side effect, which experts say is extremely rare, and to looking into whether the same types of rare clots could occur with other shots.”
In the article, vaccine expert Paul Offit, MD, Director of the Vaccine Education Center at Children’s Hospital of Philadelphia, pondered why a vaccine would lead to the production of antibodies against platelet factor 4 and suggested that it’s likely something about the adenovirus — the harmless virus that delivers the COVID-19 vaccine — that is interacting with platelet factor 4 and, in turn, causing the rare blood clotting problem.
The more researchers learn about the underlying link, the sooner we may be able to determine who’s at a greater risk for this rare side effect than others.
Herper adds that “while researchers try to figure out the biology behind the blood clots, doctors and people who need vaccines are left to balance the risks and benefits of the AstraZeneca and Johnson & Johnson vaccines with incomplete information.”
He, like Dr. Jetelina, point out that some countries are making decisions about how to allocate different COVID-19 vaccines accordingly — for example, by giving Pfizer and Moderna vaccines to people under age 30 and using the AstraZeneca vaccine in older adults.
Read the full article here.
Author Steven W. Thrasher compares the Johnson & Johnson vaccine blood clot reports to the bigger picture of COVID cases and deaths
In a straightforward tweet, Dr. Thrasher used statistics to emphasize the importance of vaccinations and severity of COVID-19.
“For scale, [please] keep in mind:
—About one in every 588 Americans have died of COVID (562K of 331 million ppl)
—About one in 10 Americans have been infected w COVID (31 million of 331 million)
—About 1 in a million who got J & J have had blood clots — just 6 ppl out of 6 million,” he wrote.
He adds that it’s hard to convey this sense of scale, but that the risk of COVID, the third-greatest killer of Americans in 2020, is “FAR more than any risk from J&J [vaccine]. Unfortunately, headlines and framing may skew the potential risk.”
He notes that the pause in the Johnson & Johnson vaccine because of the one in a million possibility of blood clots will sow doubt about how to best address a one in 588 risk of death from COVID, which only cause more death.
Read Dr. Thrasher’s full Twitter thread here.
Krutika Kuppalli, MD FIDSA, shares appreciation (and further explanation) for the Johnson and Johnson pause
In a series of Tweets, Dr. Kuppalli of the Johns Hopkins Center for Health Security, emphasized the importance of this step to ensure vaccine confidence and continuation of the vaccine rollout.
“We need to make sure our #vaccines are safe,” Kuppalli wrote. “Having transparency and quick action in this process is key and they only way we will ensure that the public has confidence in our regulatory agencies and the vaccines.”
Dr. Kupalli, like others, questioned whether the viral technology is what’s responsible for the increased risk of blood clots in both the AstraZeneca and Johnson & Johnson vaccines. (Pfizer and Moderna use a different kind of technology, and have not been linked to an increase risk of blood clots). She pondered whether there is something about the viral vector technology that could cause an immune response that leads to a “hypercoaguable” (tendency toward blood clotting) state.
If data shows that to be the case, it’s not necessarily a cause for alarm, but rather could allow public health authorities to allocate COVID-19 vaccines in ways to help mitigate this risk—for example, by giving non-viral vector vaccines to people who may be at a greater risk of clots (younger women).
Read more of Dr. Kuppalli’s tweets here, here, here, and here.
Get Free Coronavirus Support for Chronic Illness Patients
Join the Global Healthy Living Foundation’s free COVID-19 Support Program for chronic illness patients and their families. We will be providing updated information, community support, and other resources tailored specifically to your health and safety.
AstraZeneca’s COVID-19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets. European Medicines Agency. April 7, 2021. https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood.
Cerebral Venous Sinus Thrombosis (CVST). Johns Hopkins Medicine. https://www.hopkinsmedicine.org/health/conditions-and-diseases/cerebral-venous-sinus-thrombosis.
Herper M. Why would a Covid vaccine cause rare blood clots? Researchers have found clues. STAT News. April 13, 2021. https://www.statnews.com/2021/04/13/researchers-search-for-answers-in-puzzle-of-blood-clots-and-covid-vaccines-and-see-some-clues/ .
Jetelina, K. J&J: Questions answered from a scientific perspective. Your Local Epidemiologist. April 13, 2021. https://yourlocalepidemiologist.substack.com/p/j-and-j-questions-answered-from-a.
Joint Media Call: FDA & CDC to Discuss Janssen COVID-19 Vaccine. YouTube. April 13, 2021. https://www.youtube.com/watch?v=_ELXnGYgsJY.