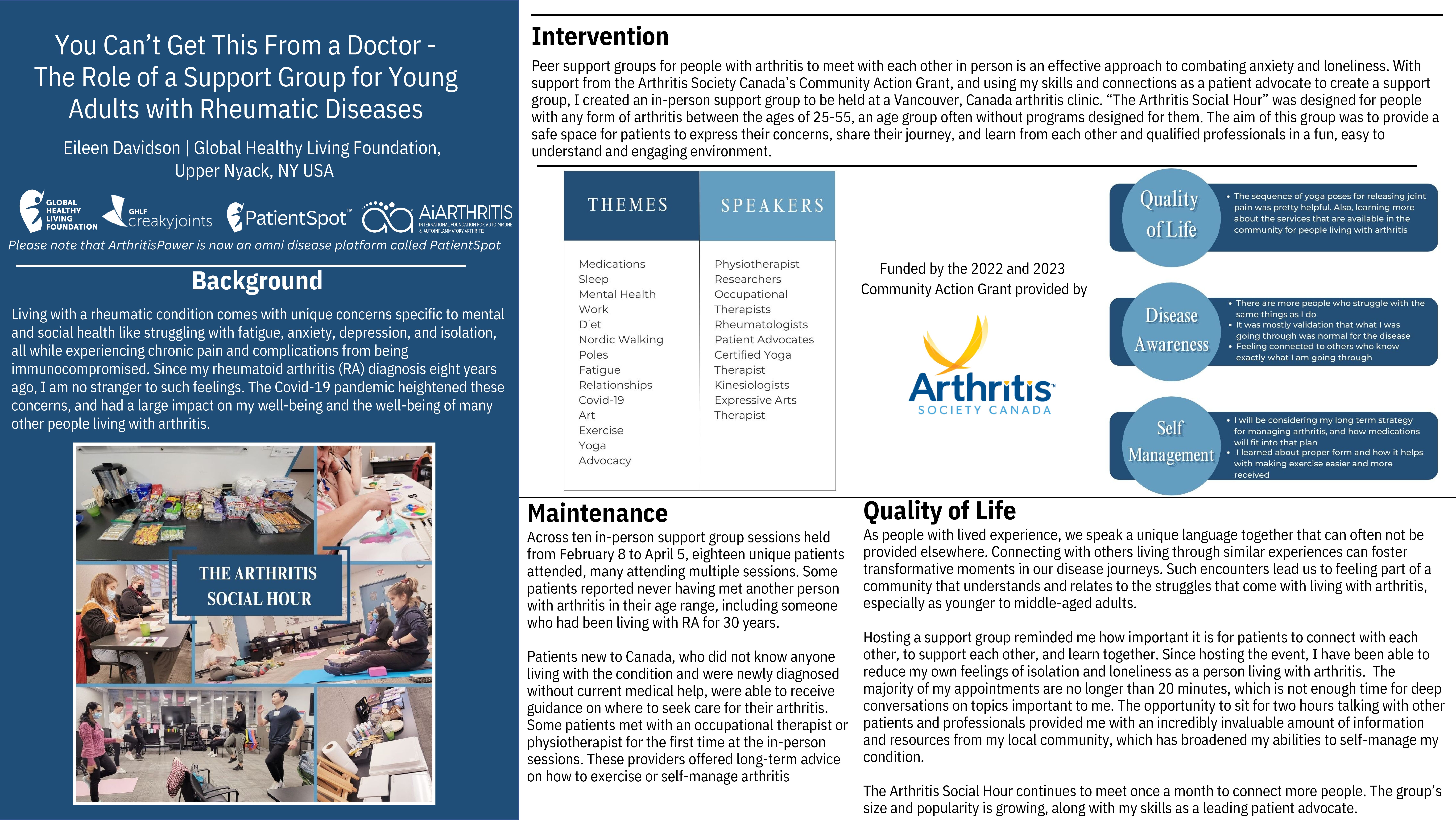
Learn more about our FREE COVID-19 Patient Support Program for chronic illness patients and their loved ones.
Have a runny nose, cough, and other questionable symptoms — but aren’t sure if it’s the flu, COVID-19, or a common cold? You may soon be able to test for both the flu and COVID-19 at once with an at-home test.
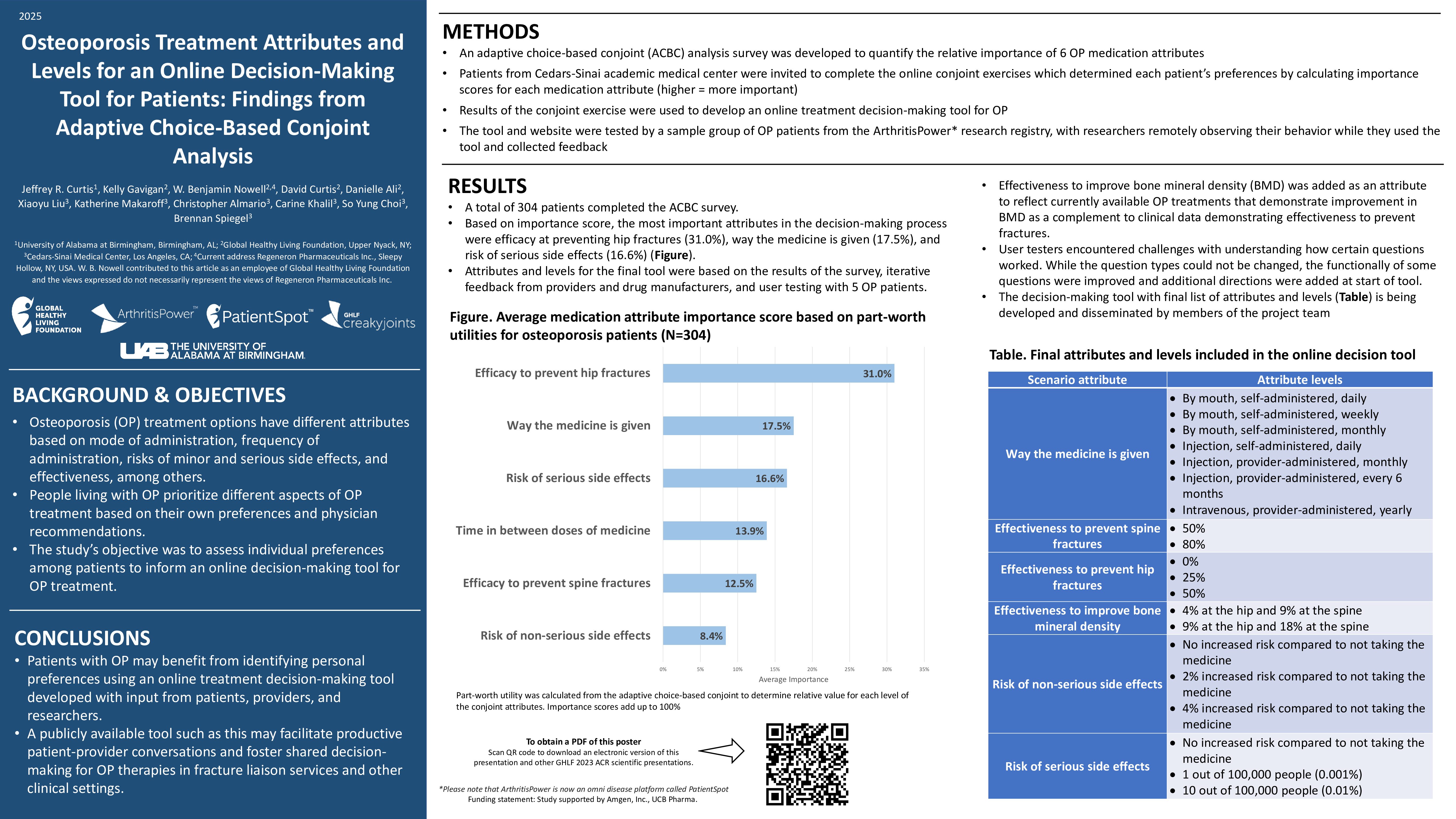
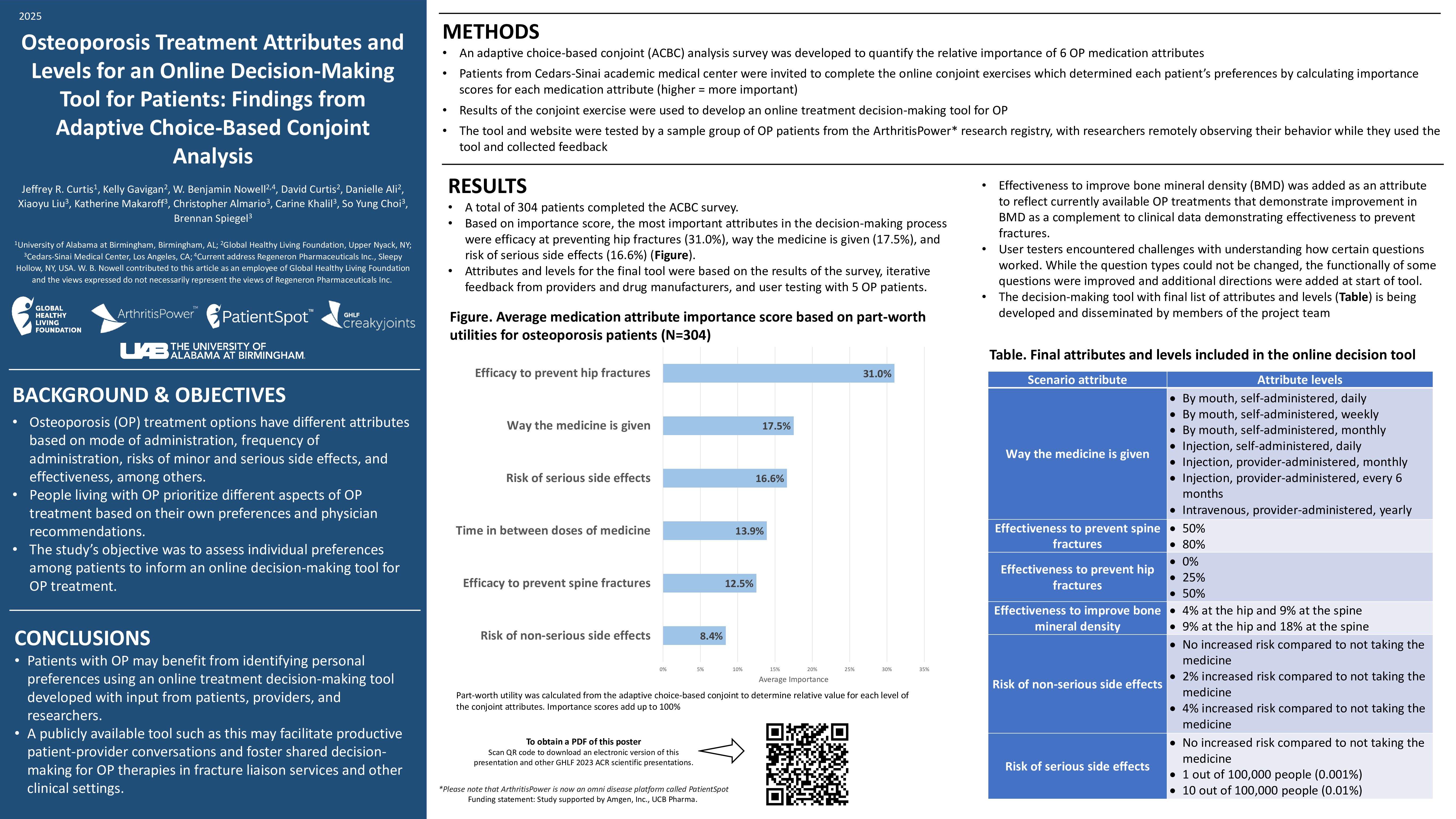
The U.S. Food & Drug Administration (FDA) recently issued an emergency use authorization for the Lucira COVID-19 & Flu Home Test, a single-use, at-home test kit that provides results from your nasal swab samples. Designed for self-collection by those ages 14 years or older, or collected by an adult for those 2 years or older, this test can be purchased without a prescription and performed completely at home, per the FDA.
Similar to a standard COVID-19 test, the new test works by swirling a sample swab in a vial that’s then placed in a test unit. In 30 minutes, it will show if you’re positive or negative for Influenza A, Influenza B, and COVID-19. (There are four broad categories of flu: A, B, C, and D. Influenza A, B, and C are the most common, but A and B are seasonal and have more severe symptoms, per the Cleveland Clinic.)
According to the FDA, the Lucira combination test correctly identified 99.3 percent of negative and 90 percent of positive Influenza A samples — and 100 percent of negative and 88.3 percent of positive COVID-19 samples.
It also correctly detected 99.9 percent of negative Influenza B samples, but there aren’t currently enough cases of Influenza B circulating to include in a clinical study. Because of that, validation confirmed that the test can detect the virus in contrived specimens. The FDA’s emergency use authorization requires Lucira to continue collecting samples to study the test’s ability to detect Influenza B in real-world settings.
The emergency authorization came just two days after Lucira Health filed for bankruptcy protection, which the company claimed was partly due to the lengthy FDA approval timeline, per The New York Times. Because of that, it remains unclear when the test will become available to Americans.
“Lucira is kind of stuck in this ironic position,” says Wilbur A. Lam, MD, PhD, a Pediatric Hematologist and Biomedical Engineer at Emory University and Georgia Institute of Technology, whose team established a National Institutes of Health (NIH)-funded center for fostering the development of point-of-care technologies, including Lucira’s new combination test. His group has helped the NIH and FDA assess not just COVID-19 tests, but also combination COVID and flu tests like Lucira’s.
“The hope is that once you have one authorization, that opens up the door for other companies as well,” adds Dr. Lam. “We don’t speak for the FDA, but our expectation is that there will be other companies coming around the bend that will also have a combination flu and COVID-19 test.”
Lucira’s at-home combination test has been available in Canada since August 2022 and currently costs around $70 per test, per CNBC. “I think that they plan to sell it for half that, but we’ll have to wait and see,” says Sarah Farmer, a Research Scientist and Director of Testing and Evaluation at the Center for Advanced Communications Policy at Georgia Tech. Farmer helps the NIH and FDA evaluate usability, accessibility, and human factors for COVID-19 and flu combination tests like Lucira’s.
“Most of the tests on the market are lateral flow antigen tests, which is much simpler technology,” Farmer adds. “The Lucira technology is molecular, so there’s just more involved there.”
Molecular tests like PCR tests detect genetic material called RNA from the virus and tend to be more accurate (but usually take longer and have to be sent into a lab), per the FDA. Antigen tests are often referred to as rapid tests and are the standard at-home COVID-19 tests you may think of, but these tend to be less accurate than molecular tests.
This Lucira combination test requires 30 minutes to process results, which may be longer than the COVID-19 antigen tests you’re used to. “This test has different scientific principles underlying its technology,” says Dr. Lam. “The company says their value proposition is that you have the performance of a PCR test, but the convenience of a rapid test, and therefore it does take a little more time for the chemical reaction to take place.”
Tips for Using an At-Home COVID-19 and Flu Test
When this test (or a similar one) becomes available for at-home use in the United States, there are a few guidelines you should follow to get the most accurate results.
“Read the instructions really carefully before you start the test and make sure you understand them,” says Farmer. “Don’t assume a test that’s new to you will be like a test you’ve done in the past. Tests are going to be different in terms of their labeling schemes, time windows, steps, and so forth.”
Get situated in a space where you can be free of distractions, focus on your test, and read the results with good lighting.
“Make sure you’re listening, feeling, or looking for feedback mentioned in the instructions, because this will help you know that you’ve done a task correctly,” says Sarah. “Something specific to Lucira is the tactile, audible click into place when you insert that vile into the test unit — and the blinking lights that let you know the test has begun processing.”
If you take the Lucira at-home combination test, keep in mind that some of the positive results may show up before the test is done processing. However, wait until the test’s light indicates that it’s time to look at your results, since some may appear more slowly.
“There are a lot of different combinations of results that you may see, so double check the instructions,” says Farmer. “Typically, you’ll see images of all possible results, so make sure you’re matching those up to make sure you understand what your results are.”
It’s rare that you’ll test positive for both the flu and COVID, but it’s possible. Of course, if you do receive any type of positive result, contact your doctor right away to discuss potential treatments (keep in mind that certain antivirals, including Paxlovid, must be taken within five days of symptom onset).
Get Free Coronavirus Support for Chronic Illness Patients
Join the Global Healthy Living Foundation’s free COVID-19 Support Program for chronic illness patients and their families. We will be providing updated information, community support, and other resources tailored specifically to your health and safety. Join now.
COVID-19 Test Basics. U.S. Food & Drug Administration. January 4, 2023. https://www.fda.gov/consumers/consumer-updates/covid-19-test-basics.
FDA Authorizes First Over-the-Counter At-Home Test to Detect Both Influenza and COVID-19 Viruses. U.S. Food & Drug Administration. February 24, 2023. https://www.fda.gov/news-events/press-announcements/fda-authorizes-first-over-counter-home-test-detect-both-influenza-and-covid-19-viruses.
Interview with Wilbur A. Lam, MD, PhD, a pediatric hematologist and biomedical engineer at Emory University and Georgia Institute of Technology.
Sarah Farmer, a research scientist and Director of Testing and Evaluation at the Center for Advanced Communications Policy at Georgia Tech.
First At-Home Combination Test for Flu and Covid Is OK’d by the F.D.A. The New York Times. February 24, 2023. https://www.nytimes.com/2023/02/24/health/covid-flu-test-fda.html.
Flu (Influenza). Cleveland Clinic. October 11, 2022. https://my.clevelandclinic.org/health/diseases/4335-influenza-flu.
The FDA has authorized the first combination at-home test for Covid and flu—here’s what to know. CNBC. March 1, 2023. https://www.cnbc.com/2023/03/01/first-fda-authorized-at-home-combination-test-for-covid-and-the-flu.html.