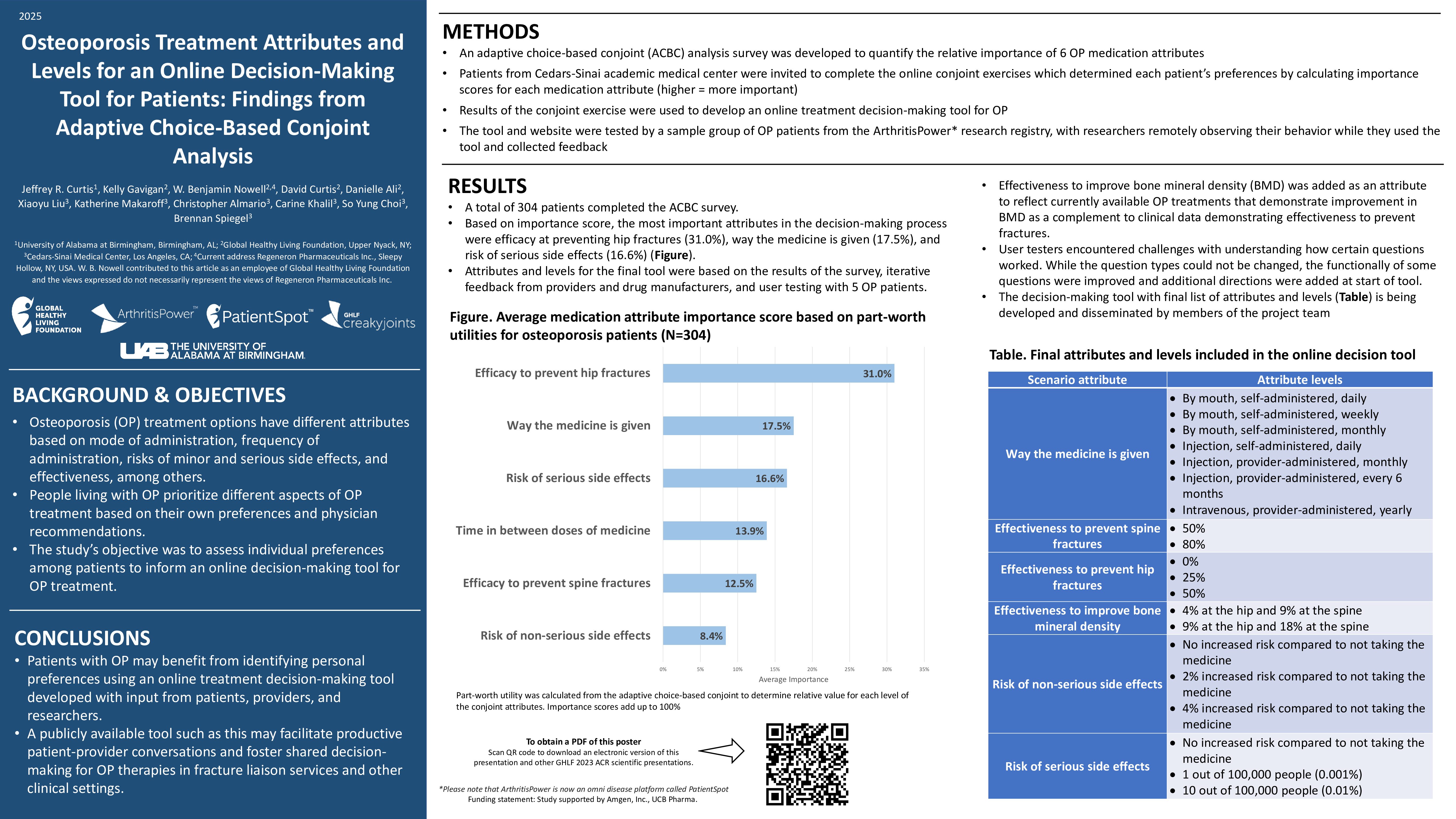
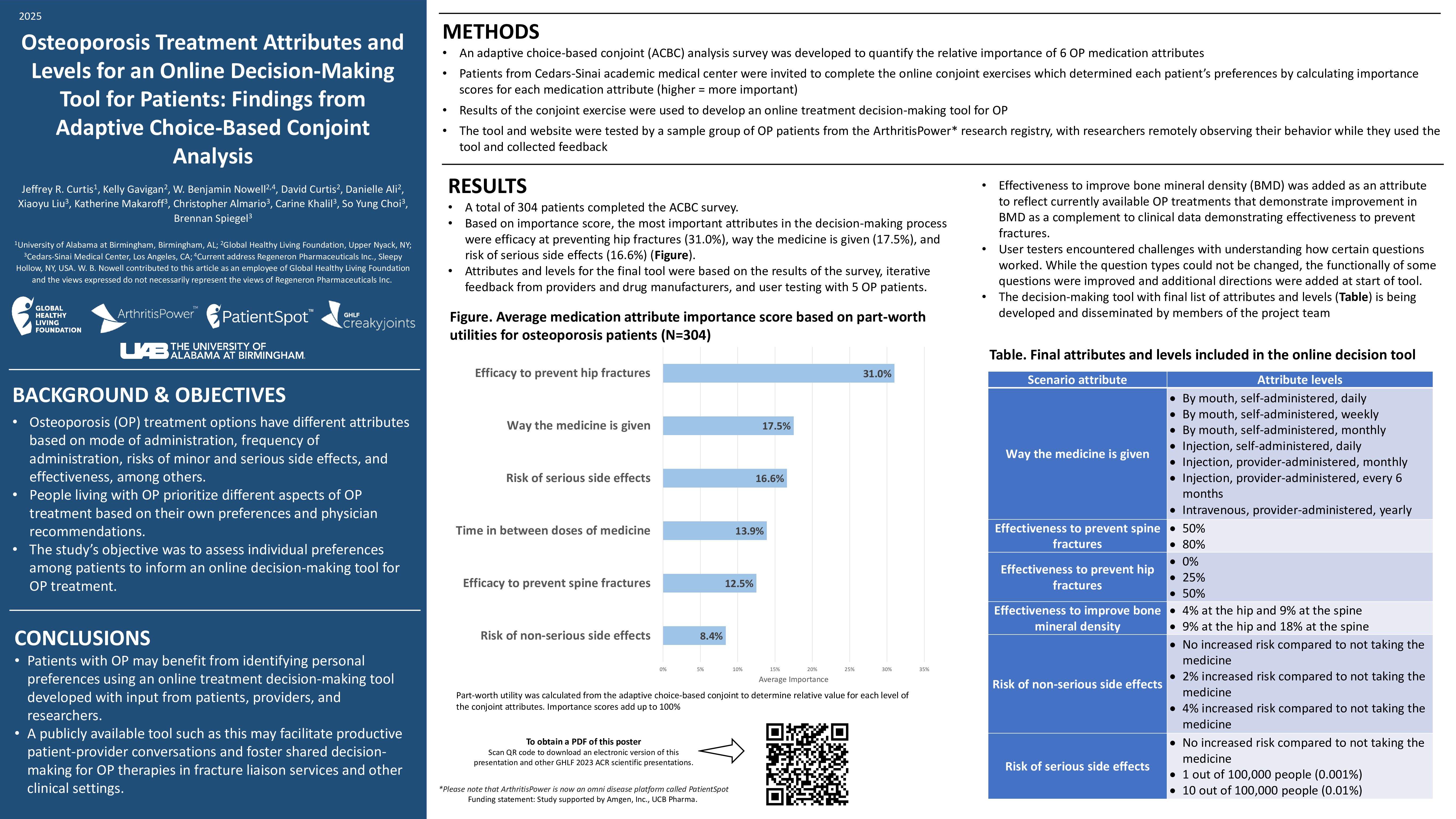
Learn more about our FREE COVID-19 Patient Support Program for chronic illness patients and their loved ones.
Throughout the pandemic, scientists have discovered that being biologically male or female might impact you when it comes to COVID-19 outcomes, long COVID, and vaccine responses — but the reasons for these differences have yet to be fully understood.
While experts investigate this topic (many are calling for more studies that look at outcomes based on sex), it’s important to know how your sex may affect your risk, particularly if you’re immunocompromised. This should be part of the ongoing conversation you have with your doctor to determine your risk level and your strategy for protecting yourself against COVID-19.
Here’s what we know about the differences in COVID-19 between males and females so far — and what it may mean for you.
Differences in COVID-19 Infection and Outcomes in Females vs. Males
While researchers agree that males die of COVID-19 at a higher rate than females, it’s not entirely clear why. A series of social factors may play a bigger role than biological differences, per a February 2022 Harvard GenderSci Lab study of more than 30 million confirmed coronavirus cases in the United States.
The team found that males were infected and died at higher rates than females in some states, but these rates were about even in other states. And at certain points during the pandemic, females outpaced males in cases and fatalities. Because of this, looking at the aggregate data for the nation could be misleading.
What’s more, the gap between males and females was smaller than what experts originally thought: Early on, it was reported that males were dying of COVID-19 at twice the rate of females. However, the data from the team’s tracker showed that males in the United States had a mortality rate that was just 10 to 20 percent higher than that of females between April 2020 and May 2021.
After statistical modeling, the researchers found that 30 percent of this variation was due to state-level factors, such as differences in public health policies, timing and length of mask mandates, and other social factors like gendered health behaviors, occupational exposures, pre-existing health conditions, and demographics including race, age, and education.
The model also showed that 10 percent of the difference was based on when a measurement was taken during the pandemic.
The remaining 60 percent of the variation was not explained by either time or state. Still, researchers don’t believe that interventions centered on sex-related biological factors — like the use of testosteron blockers or estrogen — would have a significant impact on outcomes.
“Without considering [social and contextural] factors, you’re missing part of the picture of why people might be getting exposed or getting a more severe case,” Tamara Rushovich, a graduate student at the T.H. Chan School of Public Health and part of the GenderSci Lab research team, told The Harvard Gazette. “For example, when you see numbers that show different rates of cases or deaths, it’s not just biology, but what was your exposure risk? And that’s influenced by things like your occupation or your income.”
Gendered behaviors can also play a role in the different outcomes between males and females.
“There’ve been studies that look at things like adherence to mask policies or social-distancing guidelines,” added Rushovich. “They saw differences in gender adherence to those, so men being less likely to wear masks properly or to adhere to social distancing guidelines.”
For instance, an October 2020 study in the Proceedings of the National Academy of Sciences of the United States of America looked at the gender differences in COVID-19 attitudes and behavior from eight countries. Researchers found that 59 percent of females considered COVID-19 to be a very serious problem during the first wave of the pandemic (March 16-30, 2020) across all eight countries studied (Australia, Austria, France, Germany, Italy, New Zealand, United Kingdom, and the United States), compared to just 48.7 percent of the males.
In both periods studied (including the second period from April 15-20, 2020), individuals were asked to evaluate how strictly they were following seven recommended rules, including keeping physical distance from others or staying at home. Compliance was markedly higher in females (by 5 to 6 percent), but smaller than the differences in beliefs about the pandemic.
And even after adjusting for sociodemographic characteristics and employment status, females were much more likely than males to believe that the health consequences of the pandemic were very serious — and were also more supportive of restraining measures and more compliant with public health and social distancing measures, per the researchers.
The risk of severe COVID-19 outcomes may be further heightened in certain immunocompromised populations. In an April 2021 review in the Journal for ImmunoTherapy of Cancer, researchers found that individuals who were hematopoietic stem cell transplant (HCT) recipients had a 68 percent rate of 28-day survival after COVID-19 — with risk factors for mortality including being male, being older than 50 years, and getting infected within 12 months after HCT.
Likewise, the COVID-19 Global Rheumatology Alliance (GRA) has looked at factors associated with COVID-19 mortality in 3,729 patients with rheumatic diseases. Being male, having an older age, and living with certain comorbidities (hypertension, heart disease, and chronic lung disease) were risk factors for COVID-19-related death. More rheumatic disease activity and certain medications (like rituximab) also raised risk.
Long COVID in Males vs. Females
On the other hand, some research shows that females might be more likely to encounter long COVID than males — but additional studies are needed to confirm this.
A June 2022 review in Current Medical Research and Opinion found that the likelihood of having long-COVID syndrome was 22 percent greater among females. In particular, female patients were significantly more likely to have long-COVID symptoms in the categories of psychiatric and mood (i.e. depression); ear, nose, or throat; musculoskeletal (i.e. myalgia); and respiratory.
That said, male patients were significantly more likely to have long COVID in the category of renal disorders (i.e. acute kidney injury).
“Differences in immune system function between females and males could be an important driver of sex differences in long COVID-19 syndrome,” note the researchers. “Females mount more rapid and robust innate and adaptive immune responses, which can protect them from initial infection and severity. However, this same difference can render females more vulnerable to prolonged autoimmune-related diseases.”
As with Lyme disease, the COVID-19 pathogen might remain hidden and generate greater levels of inflammatory cytokines in females than males, per a February 2022 review in the European Respiratory Journal. Some experts believe that fragments of SARS-CoV-2 could hang around areas of the body like the kidneys or brain, sparking a chronic inflammation-associated cascade. This may result in symptoms like pain or brain fog.
In general, 80 percent of all individuals affected by autoimmune disorders are females, due to variation within the sex chromosomes and hormonal changes, per a May 2020 review in Cureus. (An increasing body of research has pointed toward the possibility that COVID-19 causes the development of autoantibodies linked to other autoimmune diseases — and may be tied to long-COVID symptoms.)
All of this said, most studies on long COVID do not evaluate or report granular data by sex, so more research in this area will be key for better understanding the risk of long COVID.
“The lack of studies reporting sex-disaggregated outcomes for COVID-19 speaks to the need for further, large-scale research that includes sex as an analytical variable and that reports data by sex,” note the researchers of the Current Medical Research and Opinion review.
It’s important to talk to your doctor about your risk of long COVID as an immunocompromised patient — and how your sex might play a role in that risk.
“I have not seen data to suggest confirming that immunocompromised patients are more likely to develop long COVID than patients who are not immunocompromised,” Samoon Ahmad, MD, clinical professor of psychiatry at NYU Grossman School of Medicine, told us previously. “That said, it’s clear that immunocompromised patients are more likely to develop severe COVID if they get it — and research suggests that people who have severe COVID are more likely to develop long COVID.”
Read more about what you should know about long COVID.
COVID-19 Vaccine in Females vs. Males
Individuals who are biologically male or female also have differences when it comes to the COVID-19 vaccine, whether it’s in regards to vaccine hesitancy or risk of adverse effects.
Initially, females were more hesitant than males to get the COVID-19 vaccine. In a review of 60 studies published in the Journal of Public Health, researchers found that 58 percent of papers reported males having higher intentions to get vaccinated against COVID-19. Significantly fewer females stated that they would get vaccinated than males during the time period studied (November 2020 to January 2021).
Overall, males were on average 41 percent more likely to report that they intended to receive a vaccine — rather than being unwilling or undecided — compared with females. The gender effects were even higher among health care workers compared with unspecified population samples. (That said, this result requires cautious interpretation, given that gender proportions in the health care worker samples were highly unbalanced and the number of studies with health care worker samples was comparatively small.)
However, many of the studies included in this review asked individuals about their intentions to get vaccinated before a vaccine was available.
By April 2021, more females than males had gotten vaccinated in many states, per the Kaiser Family Foundation. The vaccine breakdown between males and females was generally close to 60 percent and 40 percent — for instance, 58 percent of those vaccinated in Alabama were females and 57 percent were females in Florida.
There may be many reasons for this difference: For instance, females make up three-quarters of the workforce in health care and education, which were sectors prioritized for initial vaccines. Females also tend to have longer life spans, so older individuals initially eligible for vaccines were more likely to be female. However, the gender gap continued even as eligibility expanded to all adults.
When it comes to rare adverse reactions to the vaccines, males and females also appear to be affected differently. In a February 2022 review in the journal Vaccines, researchers found that the risk of adverse events after the Pfizer-BioNTeach COVID-19 vaccine were consistently higher in females of all ages. This included local responses such as pain at the injection site, systemic events such as fever, and sensory events such as paresthesia (a burning, prickling sensation) in the hands and face.
Females may have increased reactogenicity of vaccines and are at higher risk of anaphylaxis, per the review.
“The remarkably consistent excess in the rates of adverse events in females following immunization with the Pfizer-BioNTech COVID-19 vaccine, in all age groups, suggests that gender-specific factors influence the response to the vaccine,” note the researchers. “These findings indicate that different doses of the vaccine for men and women should be explored.”
Females also report more vaccine side effects in general. More than 79 percent of nearly 7,000 reports processed through the Centers for Disease Control and Prevention (CDC) Vaccine Adverse Event Reporting System from December 14, 2020 to January 13, 2021 came from females, per the CDC’s Morbidity and Mortality Weekly Report. The most frequently reported symptoms were headache, dizziness, and fatigue.
This could be due to females’ greater immune response. “From a biological perspective, women and girls produce sometimes twice as many infection-fighting antibodies from vaccines,” said Rosemary Morgan, a research scientist at Johns Hopkins Bloomberg School of Public Health, told USA TODAY.
What’s more, male sex hormones like testosterone and dihydrotestosterone (DHT) have immunosuppressive qualities because of the way they modulate the breakdown of fat, per St. Luke’s Health. Some research has shown that males have lesser antibody responses and lesser inflammatory cytokine expression when given the flu shot than females.
However, this doesn’t appear to affect COVID-19 vaccine efficacy rates — which were actually slightly higher for males than females in clinical trials. For instance, clinical trials showed that the Moderna vaccine was 95.4 percent effective at preventing COVID-19 in males, compared to 93.1 percent for females. For the Pfizer vaccine, efficacy was 96.4 percent in males and 93.7 percent in females.
One study in the journal Molecular Pharmacology looked at whether fat-based nanoparticles could be the cause behind the difference in vaccine efficacy. Researchers found that there were significant differences in the uptake of these nanoparticles between male and female natural killer cells (a type of immune cell that has small particles with enzymes that can kill cells infected with a virus).
“The results of this proof-of-concept study show the importance of recipient sex as a critical factor which enables researchers to better consider sex in the development and administration of vaccines for safer and more-efficient sex-specific outcomes,” note the researchers.
What This Means for You
If you’re immunocompromised, you’re likely already mindful of mitigation efforts to protect yourself against COVID-19. While being biologically male or female isn’t likely to make a major impact on your risk of severe disease (unless you partake in behavior that increases your risk), it might affect your chances of long COVID.
And while it’s possible you may have a slightly higher risk of an adverse reaction to the vaccine if you’re female, such reactions are rare. In most cases, the benefits of the COVID-19 vaccine outweigh the risks.
Of course, as an immunocompromised patient, it’s important to be aware of every tool you have to protect yourself and to stay aware of your risks. For instance, you should have a conversation with your doctor about your risk level for long COVID should you get infected (and how your sex may play a role in that risk).
Although we still have much to learn about the differences between females and males when it comes to COVID-19, you can use the clues available to create the best-informed strategy to protect yourself in partnership with your doctor.
Get Free Coronavirus Support for Chronic Illness Patients
Join the Global Healthy Living Foundation’s free COVID-19 Support Program for chronic illness patients and their families. We will be providing updated information, community support, and other resources tailored specifically to your health and safety. Join now.
Angum F, et al. The Prevalence of Autoimmune Disorders in Women: A Narrative Review. Cureus. May 13, 2020. doi: https://doi.org/10.7759/cureus.8094.
Danielsen AC, et al. Sex disparities in COVID-19 outcomes in the United States: Quantifying and contextualizing variation. Social Science & Medicine. January 10, 2022. doi: https://doi.org/10.1016/j.socscimed.2022.114716.
Galasso V, et al. Gender differences in COVID-19 attitudes and behavior: Panel evidence from eight countries. PNAS. October 15, 2020. doi: https://doi.org/10.1073/pnas.2012520117.
Gee J, et al. First Month of COVID-19 Vaccine Safety Monitoring — United States, December 14, 2020–January 13, 2021. Morbidity and Mortality Weekly Report (MMWR). U.S. Centers for Disease Control and Prevention. February 19, 2021. doi: http://dx.doi.org/10.15585/mmwr.mm7008e3.
The Gender Vaccine Gap: More Women Than Men Are Getting Covid Shots. Kaiser Family Foundation. April 12, 2021. https://khn.org/news/article/gender-vaccine-gap-more-women-than-men-vaccinated-against-covid/.
Goldman JD, et al. COVID-19 in immunocompromised populations: implications for prognosis and repurposing of immunotherapies. Journal for ImmunoTherapy of Cancer. April 27, 2021. doi: https://doi.org/10.1136/jitc-2021-002630.
Green MS, et al. Gender Differences in Adverse Events Following the Pfizer-BioNTech COVID-19 Vaccine. Vaccines. February 3, 2022. doi: https://doi.org/10.3390/vaccines10020233.
Ortona E, et al. Long COVID: to investigate immunological mechanisms and sex/gender related aspects as fundamental steps for tailored therapy. European Respiratory Journal. February 3, 2022. doi: https://doi.org/10.1183/13993003.02245-2021.
7 Reasons Why Men and Women React Differently to COVID-19 and the Vaccines. St. Luke’s Health. May 13, 2021. https://www.stlukeshealth.org/resources/7-reasons-why-men-and-women-react-differently-to-covid-19-and-the-vaccines.
Sylvester SV, et al. Sex differences in sequelae from COVID-19 infection and in long COVID syndrome: a review. Current Medical Research and Opinion. June 20, 2022. doi: https://doi.org/10.1080/03007995.2022.2081454.
Vulpis E, et al. The Possible Role of Sex As an Important Factor in Development and Administration of Lipid Nanomedicine-Based COVID-19 Vaccine. Molecular Pharmacology. June 7, 2021. https://doi.org/10.1021/acs.molpharmaceut.1c00291.
Women report more side effects from the COVID-19 vaccine than men. Health experts explain why. USA TODAY. April 11, 2021. https://www.usatoday.com/story/news/health/2021/04/10/covid-vaccine-women-report-more-side-effects-than-men-heres-why/7139366002/.
Why do more men die of COVID? It’s likely not what you think. The Harvard Gazette. January 20, 2022. https://news.harvard.edu/gazette/story/2022/01/harvard-study-looks-at-covid-19-sex-disparities/.
Zintel S, et al. Gender differences in the intention to get vaccinated against COVID-19: a systematic review and meta-analysis. Journal of Public Health. January 7, 2022. doi: https://doi.org/10.1007/s10389-021-01677-w.